
AABB's Leadership Certificate in Blood Banking and Transfusion Medicine is a key program to train current and emerging laboratory supervisors with the specific core concepts necessary to lead a successful lab. The program was developed to address shortages of laboratory supervisors in the field by educating the next generation in an engaging, self-paced certificate program.
AABB wishes to thank QuidelOrtho for its generous support of this important workforce initiative.

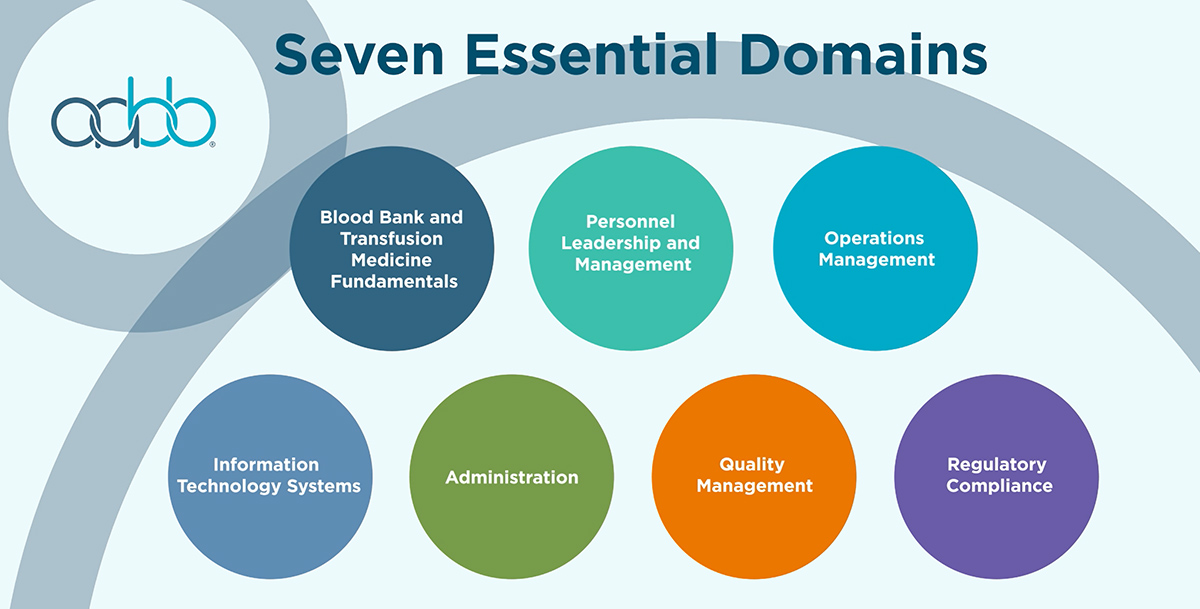
The certificate program is presented in 27 modules across seven domains, plus additional exercises and resources. A complete syllabus including learning objectives is available.
This program is offered entirely online – independent and self-paced – through AABB's Education Platform. Learners must complete all domains, assessments and a program evaluation within the year to receive the Certificate of Completion. If learners are unable to complete the program within the one-year period and still wish to complete the program, they must either re-purchase the program at full price if access has been removed or submit a retake form (includes a reduced fee of $79), which will provide an additional 12 months of access and two additional attempts on any failed domain assessment(s).
Purchase of individual domains/modules is not permitted.
Individual Registration Pricing:
Institutional Registration Pricing:
Assessment Retake Fee:
Individuals can register online via the AABB Store. If you do not have an account, you will be prompted to create one. Payment confirmation and access to the program will be provided immediately after registering.
Institutions that want to purchase registrations for learners must complete and return the Institutional Registration Form. Payment confirmation will be provided within 2-5 business days.
Discounted Rate for Learners in Low-Income and Lower-Middle-Income Countries
| Member* | Nonmember | |
| Low-Income Resident | $352 | $464 |
| Lower-Middle-Income Resident | $527 | $695 |
*Residents of qualifying countries may be eligible for a discounted AABB Membership. Join now or contact membership@aabb.org.
Low-Income/Lower-Middle-Income Institutional Registration Pricing
How to Qualify
Only professionals living and working in or facilities/organizations based in countries classified as Low-Income or Lower-Middle-Income Economies by the World Bank are eligible for the discounted registration fee. Eligible countries are based on those defined as Low-Income and Lower-Middle-Income Economies by the World Bank. Please visit the World Bank website to view the current list of qualifying Low-Income and Lower-Middle-Income countries (listing appears at the bottom of the page).
Supporting documentation must be submitted and verified by AABB (see applicable registration form for details). If the documentation does not meet requirements for the Low-Income or Lower-Middle-Income developing country discount, AABB reserves the right to change the registration to the appropriate fee and registration will not be confirmed until the balance is paid. Participation in this special discount program is not automatically guaranteed or renewed each year. No other discounts or promotional offerings will be applicable or applied.
How to Register
Low/Lower-Middle-Income Residents – download the registration form, complete and submit with payment and required documentation.
Institution/Facility Residents – download Low/Lower-Middle-Income Institutional registration form, complete and submit with payment and required documentation.
Domain 1: Blood Banking and Transfusion Medicine Fundamentals
This domain focuses on ensuring the safe and effective use of blood products in transfusion medicine. This includes identifying blood types and antibodies to prevent mismatches, selecting appropriate blood products for patients, and following regulations and standards for collection, storage and transfusion. This domain also covers patient and recipient communication, including explaining the blood donation and transfusion process and notifying donors, recipients and their families regarding abnormal findings in a manner accessible to non-professionals. This domain is broken into four modules:
Domain 2: Quality
This domain covers the leader's role in ensuring quality and safety in a blood bank or transfusion medicine setting. Learners will explore conducting root cause analyses, implementing corrective actions, directing safety training and conducting internal assessments to improve quality systems. Additionally, the Quality domain involves tracking and evaluating corrective actions, managing non-conforming products and ensuring compliance with relevant guidelines and regulations such as AABB, CLIA and FDA. This domain is broken into six modules:
Domain 3: Regulatory Compliance
This domain involves ensuring compliance with regulatory requirements and standards in the blood banking and transfusion medicine environment. Topics include registering and updating FDA licenses, implementing process changes and reporting them to regulatory bodies and ensuring current practices align with regulations. The domain also covers identifying and implementing new regulatory requirements, ensuring staff competence, overseeing supplier audits and managing product usage in accordance with SOPs and manufacturer instructions, among other responsibilities. This domain is broken into three modules:
Domain 4: Operations Management
The Operations Management domain focuses on ensuring efficient blood inventory management, staff scheduling, assessing risk and preparing for disaster, and qualifying and maintaining equipment in blood banking or transfusion medicine settings. Tasks discussed include tracking inventory, adjusting blood product levels and scheduling staff to maintain efficient workflow. It also includes developing disaster plans, drafting policies, and ensuring training and competency. Additionally, this domain discusses applying standard manufacturing techniques to blood products and ensuring validations and testing are completed. This domain is broken into three modules:
Domain 5: Administration
The Administration domain discusses managing administrative functions in a blood banking and transfusion medicine facility. This includes managing vendor and customer relationships and contracts, ensuring expenses align with the operating budget, and recommending capital investments based on analysis and operational goals. Additionally, it involves completing billing and invoice procedures, identifying operational risks, and developing risk mitigation strategies. This domain also covers ensuring facility maintenance and safety standards are met. This domain is broken into three modules:
Domain 6: Information Technology (IT) Systems
The Information Technology Systems domain covers how to manage Information technology systems and processes in a blood banking and transfusion medicine facility. This includes working with IT to establish and maintain a reporting system that meets regulatory requirements and supports operational evaluation. Additionally, it involves ensuring compliance with electronic record management and document control policies, identifying IT needs, and coordinating solutions. This domain also covers developing and testing downtime protocols for Laboratory Information Systems (LIS) or Blood Establishment Computer Systems (BECS). This domain is broken into three modules:
Domain 7: Personnel Leadership and Management
The Personnel Leadership and Management domain discusses building strong relationships, managing teams and developing personnel. This domain involves fostering relationships with stakeholders, managing teams to meet objectives, and evaluating team members for competency and career development. It also includes coordinating student rotations, mentoring new employees and conducting effective employment interviews. Additionally, this domain covers leading team meetings, ensuring staff education and certification, managing employee retention and labor rights and resolving conflicts, among other people leadership competencies. This domain is broken into five modules:
Read and study all materials for each module and complete each activity as presented. Follow the modules in order as information builds upon preceding lessons. Since this is a self-paced program, learners may decide how much time is needed to review and study the materials. It is estimated that it will take approximately 25-35 hours to complete the program. A suggested strategy is to create a study plan or timeline for completing each domain. Follow that plan to ensure timely completion within the year that you will have access to the program.
For successful completion of the program resulting in conferral of the AABB Leadership Certificate in Blood Banking and Transfusion Medicine:
This program is eligible for 30 continuing education credits/contact hours for California Nurses, California Lab Personnel, Florida Lab Personnel and General Participation credit, which can be used toward ASCP maintenance of certification. The number and type of credits awarded for this program was determined by the estimated program completion time. This program is not eligible for continuing medical education (CME) credit. For more information on each credit type, please visit the AABB Continuing Education Credits webpage. A continuing education certificate of completion will be immediately provided to learners upon reviewing all seven domains and their applicable modules, successful completion of all domain assessments, completion of the program evaluation and claiming your continuing education credit type.
While there are numerous participants that have brought this program to fruition, key faculty include (titles and affiliations at the time of program development):
Steven M. Armstrong, DHA, MLS(AMT), SBB(ASCP), FACHE
Senior Director, Reference Laboratories and Transfusion Services – NE Division
Vitalant
Karafa S.W. Badjie, MS, MLS (ASCP) SBB
Transfusion Service Manager
Mayo Clinic
Suzanne H. Butch, MLS(ASCP)CM, SBBCM, DLMCM
Pathology Administration
Michigan Medicine
Celia P. Clifford
Senior Vice President
Quality Safety, Regulatory Affairs I Service Delivery
American Red Cross Biomedical Services Headquarters
Edward Griffin, MBA, MS, CLS, MLS(ASCP)SBB, CQA(ASQ), PMP
Director, Transfusion Medicine
Department of Pathology and Laboratory Medicine
Ronald Reagan UCLA Medical Center
Daniela Hermelin, MD, CABP
Chief Medical Officer
ImpactLife
Assistant Professor of Pathology
Saint Louis University School of Medicine
Mary A. Lieb, MT(ASCP)SBB, CQA(ASQ)
Independent Technical and Quality Consultant
Alicia Prichard, MBA, MT(ASCP)SBB
Senior Vice President Biologics, Laboratories and Supply Chain
OneBlood, Inc.
Linda Rockwood, MS(CLM), MLS (ASCP)cm, CQA(ASQ)
Director of Laboratory Services
Anna Jaques Hospital
Jean Stanley, MBA, MLS(ASCP)SBB, CMQ/OE(ASQ)CQA
Global Medical Affairs Lead, Donor Screening
Roche Diagnostic Solutions
Ricardo Sumugod, MS, MLS(ASCP)SBB
Director, Blood Bank and Esoteric Testing
Systemwide Laboratory Administration
Northwestern Memorial Healthcare
Quynh Vantu, CSQE, CQA, CMDA, PMP, CPHIMS, CHTS-CP-IM, FACHE
Director of Operations
Department of Veterans Affairs
Susan M. Wilson, MLS(ASCP)SBB
Independent Consultant
Daniel Alvarado
Lab Manager
New York Presbyterian Hospital
Steven M. Armstrong, DHA, MLS(AMT), SBB(ASCP), FACHE
Senior Director, Reference Laboratories and Transfusion Services – NE Division
Vitalant
Terri Craddock, MBA, MLS(ASCP), HP
Director
Inova Blood Services
Melissa Cushing, MD
Director of Transfusion Medicine and Cellular Therapy
Weill Cornell Medicine
Vonya Drinnon, BS, MT(ASCP)
Reference Lab Administrator, Lab Technical Manager
Blood Assurance
Marlene Feliciano
Blood Bank Supervisor
Holyoke Medical Center
Edward Griffin, MBA, MS, CLS, MLS(ASCP)SBB, CQA(ASQ), PMP
Director, Transfusion Medicine
Department of Pathology and Laboratory Medicine
Ronald Reagan UCLA Medical Center
Brenda Grossman, MD, MPH
Prof of Pathology and Immunology, Prof. of Hematology
Washington University, St Loius
Cindy Ingold, MT(ASCP)SBB, CQA(ASQ)
Transfusion Medicine Manager
Barnes Jewish
Tina Ipe, MD
Associate Professor and Medical Director
UAMS Blood Bank & Transfusion Division
Nick Lilly
Compliance and Regulatory Affairs Manager
Inova Blood Donor Services, VA
Rami Nemeh
Vice President, Chief Operating Officer
Miller – Keystone Blood Center
Susan Noone, MPH, CQA(ASQ)
Regional Director
Vitalant
Adrian O'Neal
Immunohematology Reference Laboratory Manager
Miller-Keystone Blood Center
Melissa Pearl, MBA
Director, Hospital Services and Manufacturing
Blood Bank of Alaska
Melanie Sloan
Senior Director, Accreditation and Quality
Association for the Advancement of Blood and Biotherapies (AABB)
Sharon Carayiannis, MT(ASCP)HP
Vice President, Accreditation, Standards, & Quality
Tracie Nichols, MS, MLS(ASCP)CM, SBB
Director, Compliance and Domestic Outreach
Karen Palmer, MT(ASCP), CQA(ASQ)
Director, Regulatory Affairs
Faiqa Sadique, MS, SBB, MT(ASCP), CQA(ASQ)
Director, CT Programs and Global Outreach
Melanie Sloan, MS, MT(ASCP) SBB, CQA(ASQ)
Senior Director, Accreditation and Quality
Matthew Swingholm, MS, MLS(ASCP)SBBCM
Project Development Manager
A leading instructional design company, Canadian Management Centre (CMC), was engaged to create the interactive eLearning experience. CMS was also responsible for the development of the AABB and Canadian Blood Services Individual Donor Assessment modules.
AABB wishes to thank QuidelOrtho for its contribution to the field including their generous support of workforce initiatives and this program.
Learn more about Dr. Philip Levine’s legacy and contributions to transfusion medicine.