Human cells, tissues, and cellular and tissue-based products (HCT/Ps) that do not meet all the criteria in 21 CFR 1271.10(a) are regulated as drug, device and/or biological products under Section 351 of the Public Health Service Act and are commonly referred to as "351 products." For these HCT/Ps regulated as biological products, the FDA requires premarket review of safety and efficacy data before these cellular therapy products are introduced into commercial market. Some examples of HCT/Ps regulated as biological products are listed below:
Allogeneic peritoneal membrane product in guided bone regeneration and guided tissue regeneration dental procedures
Fascia lata allograft used for the repair of Anterior Cruciate Ligament defects
Allogeneic, cryopreserved vein or artery that has not been decellularized and is used for arteriovenous access during hemodialysis
Allogeneic processed acellular dermis, rolled or folded to serve as a structural support and placed in defects following breast conservation treatment
Demineralized bone matrix for repair, reconstruction, replacement or supplementation of cartilage
Allogeneic retinal pigment epithelium and neurosensory cell layer for the treatment of patients with retinal degenerative diseases
Allogeneic adipose-derived stem cells seeded onto a bone scaffold for filling, augmenting or repair of pathologically or surgically created bony voids
Allogeneic placental-derived extracellular matrix and hematopoietic progenitor cells contained in the placental vasculature of the same donor used for repair, replacement and/or reconstruction of bone defects
Generally, biological products first are studied in animals to determine their safety profile. Once adequate nonclinical safety data has been collected, the sponsor may submit an Investigational New Drug (IND) application to study the effects of the biological product in humans. If the clinical studies in humans demonstrate the biological product is safe and effective and the relative benefits outweigh the risks, the sponsor may pursue licensure by submitting a Biologics License Application (BLA). A brief overview of the phases of the regulatory development pathway for HCT/Ps regulated as biological products is shown below.
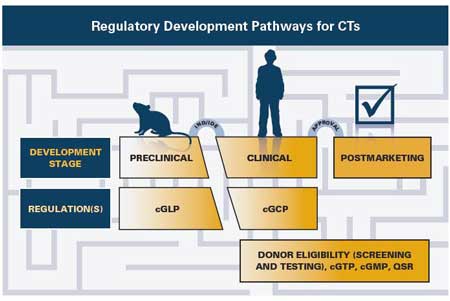 In addition to the regulatory requirements for HCT/Ps, biological products are subject to Current Good Manufacturing Practice (CGMP) during the investigational and marketing
phases of product development. Once the biological product has been licensed, it also is subject to postmarketing adverse event reporting. Topics that are discussed further include:
In addition to the regulatory requirements for HCT/Ps, biological products are subject to Current Good Manufacturing Practice (CGMP) during the investigational and marketing
phases of product development. Once the biological product has been licensed, it also is subject to postmarketing adverse event reporting. Topics that are discussed further include:
Current Good Manufacturing Practices
Labeling
Postmarketing Adverse Experience Reporting

 Effective Oct. 20, 2011, minimally manipulated hematopoietic progenitor cells (HPC) sourced from placental/umbilical cord blood for unrelated allogeneic use
require a BLA prior to being marketed, or must be distributed under an IND. These HPC, Cord Blood products also must be intended for hematopoietic reconstitution in patients with specific hematological malignancies, certain lysosomal storage and peroxisomal
enzyme deficiency disorders. The FDA has published and subsequently updated two guidance documents, "Guidance for Industry: Minimally Manipulated, Unrelated Allogeneic Placental/Umbilical Cord Blood Intended for Hematopoietic Reconstitution for Specified Indications," March 2014; and, "Guidance for Industry and FDA Staff: Investigational New Drug Applications for Minimally Manipulated, Unrelated Allogeneic Placental/Umbilical Cord Blood Intended for Hematopoietic Reconstitution for Specified Indications," March
2014, addressing the regulatory requirements for BLA and IND applications, respectively.
Effective Oct. 20, 2011, minimally manipulated hematopoietic progenitor cells (HPC) sourced from placental/umbilical cord blood for unrelated allogeneic use
require a BLA prior to being marketed, or must be distributed under an IND. These HPC, Cord Blood products also must be intended for hematopoietic reconstitution in patients with specific hematological malignancies, certain lysosomal storage and peroxisomal
enzyme deficiency disorders. The FDA has published and subsequently updated two guidance documents, "Guidance for Industry: Minimally Manipulated, Unrelated Allogeneic Placental/Umbilical Cord Blood Intended for Hematopoietic Reconstitution for Specified Indications," March 2014; and, "Guidance for Industry and FDA Staff: Investigational New Drug Applications for Minimally Manipulated, Unrelated Allogeneic Placental/Umbilical Cord Blood Intended for Hematopoietic Reconstitution for Specified Indications," March
2014, addressing the regulatory requirements for BLA and IND applications, respectively.