Cellular Therapies Section
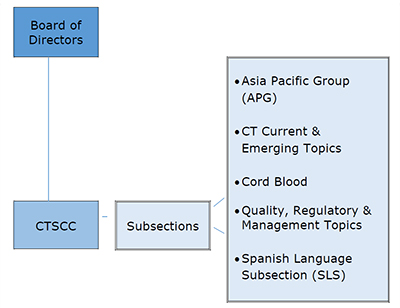 The Cellular Therapies Section was established in 2009 to provide guidance to the association and its membership
on critical issues relating to cellular therapies, including umbilical cord blood, regenerative medicine and somatic cells. The Cellular Therapies Section is guided by a Coordinating Committee, or CTSCC, comprised of elected members with cellular
therapy expertise, that ensure that the Section meets the charges described below. The Chair of the CTSCC serves on the Board of Directors. Under the leadership of members of the CTSCC, subsections have been established. The subsections are comprised
of AABB members who have indicated their interest in subsection topics.
The Cellular Therapies Section was established in 2009 to provide guidance to the association and its membership
on critical issues relating to cellular therapies, including umbilical cord blood, regenerative medicine and somatic cells. The Cellular Therapies Section is guided by a Coordinating Committee, or CTSCC, comprised of elected members with cellular
therapy expertise, that ensure that the Section meets the charges described below. The Chair of the CTSCC serves on the Board of Directors. Under the leadership of members of the CTSCC, subsections have been established. The subsections are comprised
of AABB members who have indicated their interest in subsection topics.
This page includes information on the section charges. Please follow the links to learn more about the guidelines of the Cellular Therapies Section, the subsections
and how to join them, and the current members of the CTSCC.
Charges of Cellular Therapies Section
- Link members and resources within the cellular therapy field to connect members engaged in common practices for the purpose of expertise consultation and shortening development timelines.
- Develop professional educational programs, learning tools and publications for the purpose of recruiting individuals to cellular therapy (including physicians, technologists and related health care providers).
- Advance the organization’s mission and strategic plan relating to umbilical cord blood, regenerative medicine and somatic cells.
- Identify and circulate news regarding emerging and state-of-the-art practices and applications in cellular therapy and regenerative medicine.
- Expand public awareness and education, including Web site offerings.
- Engage cell therapy professionals in interactive exchange to enhance competency.
- Obtain, analyze and disseminate up-to-the-moment summaries of regulatory guidelines and legislation related to cellular therapies.
- Facilitate the application of quality management principles to cellular therapy.
- Provide input to the CT Standards Committee regarding standards and assist in expanding the number of AABB-accredited facilities as well as providing recommendations about which novel cellular therapy products should be considered in standards development.
Cellular Therapies Section Guidelines
Cellular Therapies Section Subsections
Cellular Therapies Section Coordinating Committee Roster
Frequently Asked Questions (FAQs) for Cellular Therapies Members
Center for Cellular Therapies
 The Cellular Therapies Section was established in 2009 to provide guidance to the association and its membership
on critical issues relating to cellular therapies, including umbilical cord blood, regenerative medicine and somatic cells. The Cellular Therapies Section is guided by a Coordinating Committee, or CTSCC, comprised of elected members with cellular
therapy expertise, that ensure that the Section meets the charges described below. The Chair of the CTSCC serves on the Board of Directors. Under the leadership of members of the CTSCC, subsections have been established. The subsections are comprised
of AABB members who have indicated their interest in subsection topics.
The Cellular Therapies Section was established in 2009 to provide guidance to the association and its membership
on critical issues relating to cellular therapies, including umbilical cord blood, regenerative medicine and somatic cells. The Cellular Therapies Section is guided by a Coordinating Committee, or CTSCC, comprised of elected members with cellular
therapy expertise, that ensure that the Section meets the charges described below. The Chair of the CTSCC serves on the Board of Directors. Under the leadership of members of the CTSCC, subsections have been established. The subsections are comprised
of AABB members who have indicated their interest in subsection topics.